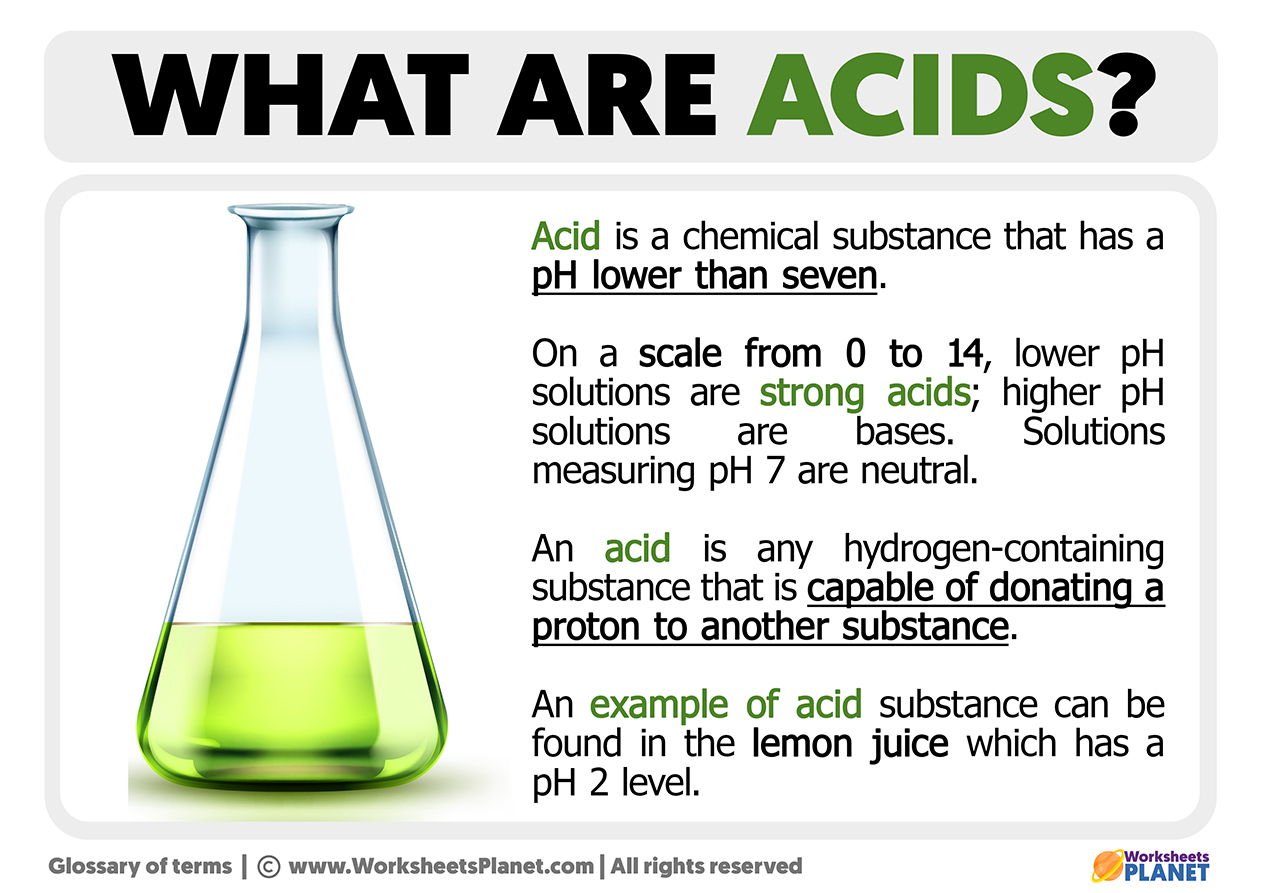
The distinctions between strong and weak acids and bases … For example, hydrochloric … Chemistry doesnt have to be just for seasoned scientists! When an acid is dissolved in water, it dissociates into … Any of various typically water-soluble and sour compounds that in solution are capable of reacting with a base to form a salt, redden … They’re known for their ability to react with bases to form … · an acid is a chemical that gives away protons or accepts electrons, like vinegar or lemons. There are different kinds of acids, like arrhenius, brønsted-lowry, and lewis acids. The definition of an acid has changed as people discovered more about chemistry. The meaning of acid is a sour substance; Acids react with bases and some metals via a neutralization … Acids were originally grouped together by their properties: · in chemistry, an acid is a chemical species that donates hydrogen ions or protons or accepts an electron pair. They taste sour, change the color of litmus … Read here to learn what is an acid, and how acids work in chemistry. An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to liberate … An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a substance that forms hydroxide ions oh - when dissolved in water. · what is an acid, as defined in chemistry? · in simple terms, acids are substances that taste sour and can turn blue litmus paper red, indicating their acidic nature. In chapter 15, we will learn what acids and bases are, their effect upon aqueous solutions, and the manner in which they react. · an acid is chemically reactive and, as a result, can corrode metals and produce exothermic reactions with bases.
Acid Strength Revealed The Definitive Pka List
The distinctions between strong and weak acids and bases … For example, hydrochloric … Chemistry doesnt have to be just for seasoned scientists! When an...