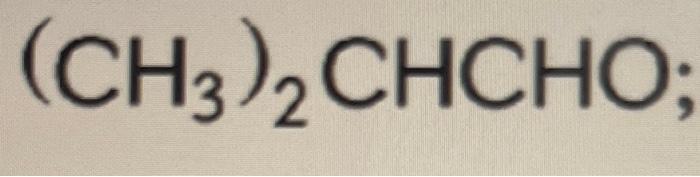
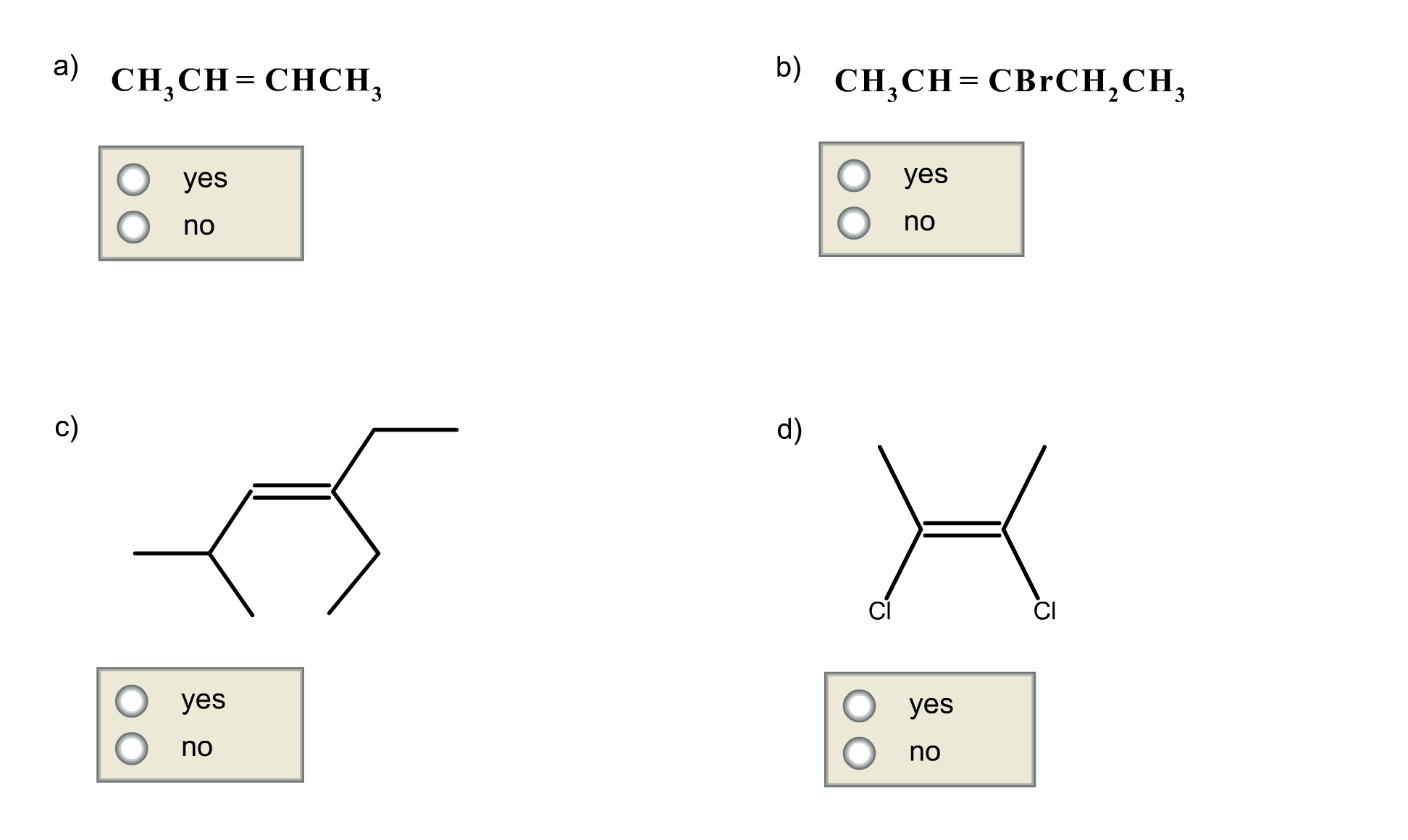
Ir spectroscopy identifies functional groups based on their characteristic absorption frequencies. Give a simple chemical test to distinguishbetween the following pair of compounds :ch3ch2cho and ch3ch2coch3 … Ch3ch2cho (propanal) and … Step by step video, text & image solution for ch_ (3)coch_ (3) and ch_ (3)ch_ (2)cho can be distinguished by : · carboxylic acids (ch₃ch₂cooh) are more acidic due to the presence of a carboxyl group (-cooh), while aldehydes (ch₃ch₂cho) … Study with quizlet and memorize flashcards containing terms like alkane, alkene, alkyne and more. Ch 3 ch 2 ch 3 shows only weak van der waal forces. In ch3ch2cho, also known as propanal, the carbonyl group (c=o) provides a site for hydrogen bonding with water. Ch 3 och 3 has some strong dipole-dipole interactions. In contrast, ch3ch2ch3, … By chemistry experts to … Ir spectroscopy can distinguish between propanal (ch3ch2cho) and propanone (ch3coch3) by analyzing the … Hence, the boiling point of …
Ch3Ch2Ch3 Vs Ch3Ch2Cho Key Differences Explained
Ir spectroscopy identifies functional groups based on their characteristic absorption frequencies. Give a simple chemical test to distinguishbetween the following pair of compounds :ch3ch2cho and...